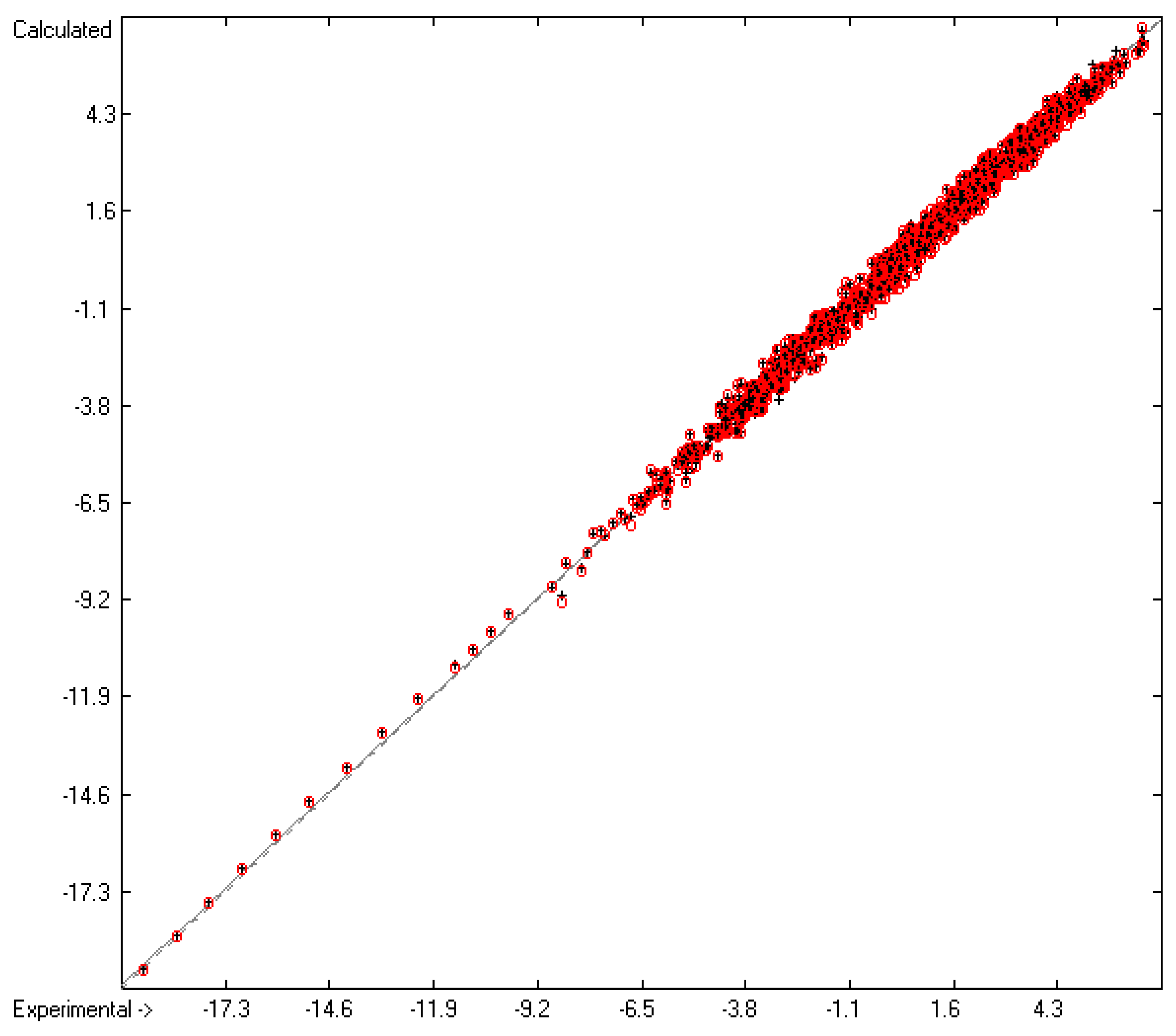
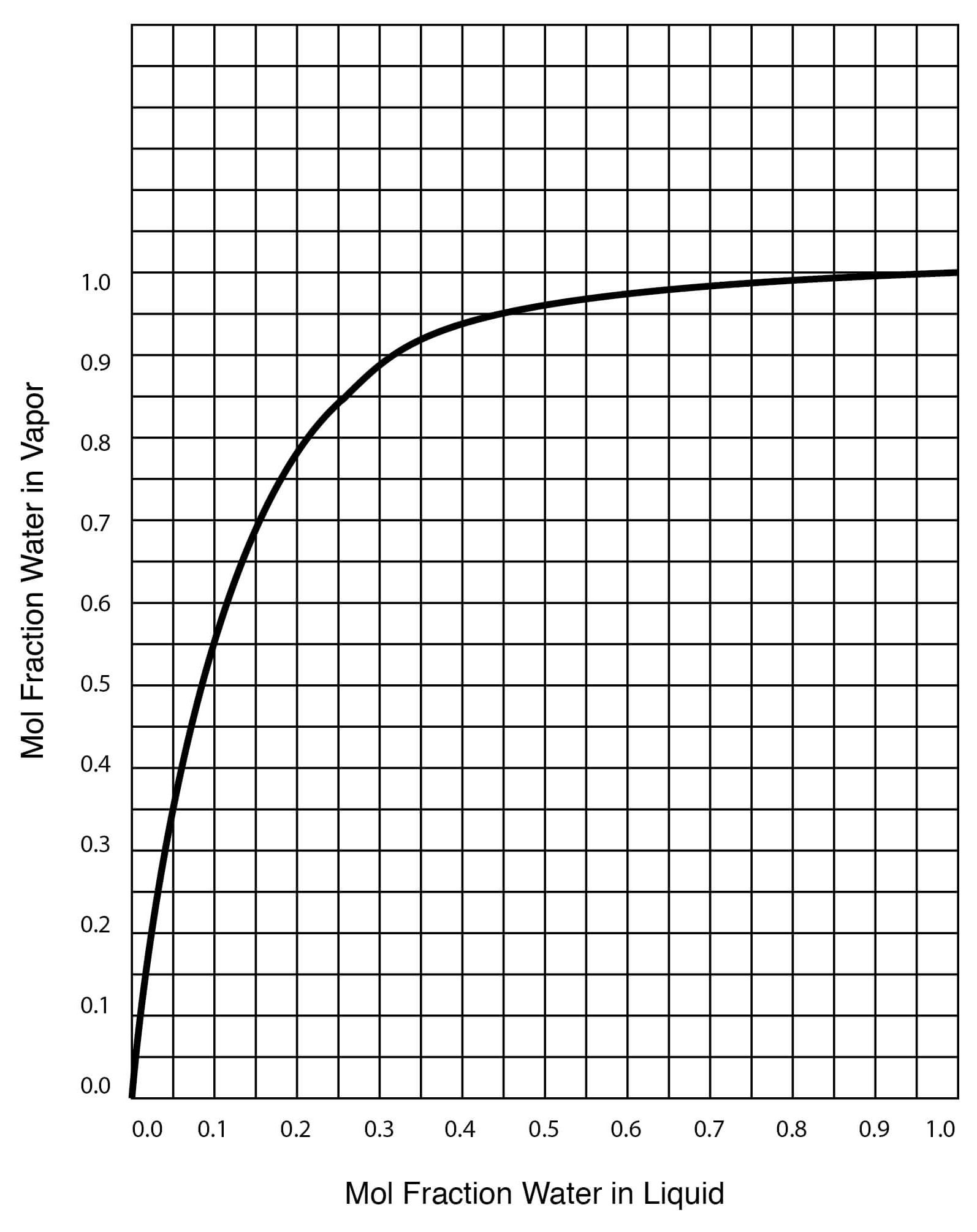
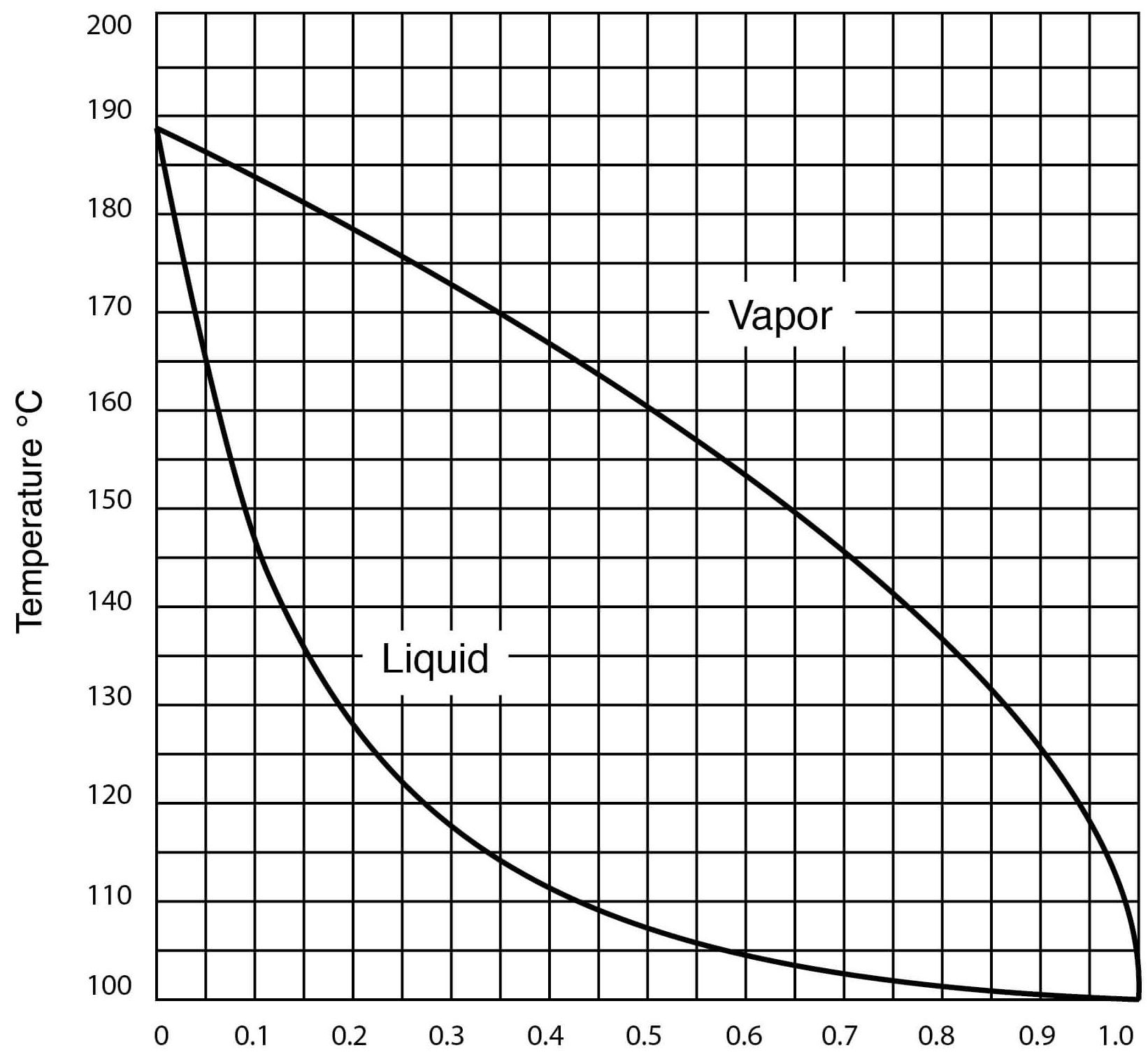
The theoretical saturation vapour pressure of butanol, water and DMSO... | Download Scientific Diagram

Vapor Pressures and Thermophysical Properties of Dimethoxymethane, 1,2-Dimethoxyethane, 2-Methoxyethanol, and 2-Ethoxyethanol: Data Reconciliation and Perturbed-Chain Statistical Associating Fluid Theory Modeling | Journal of Chemical & Engineering Data

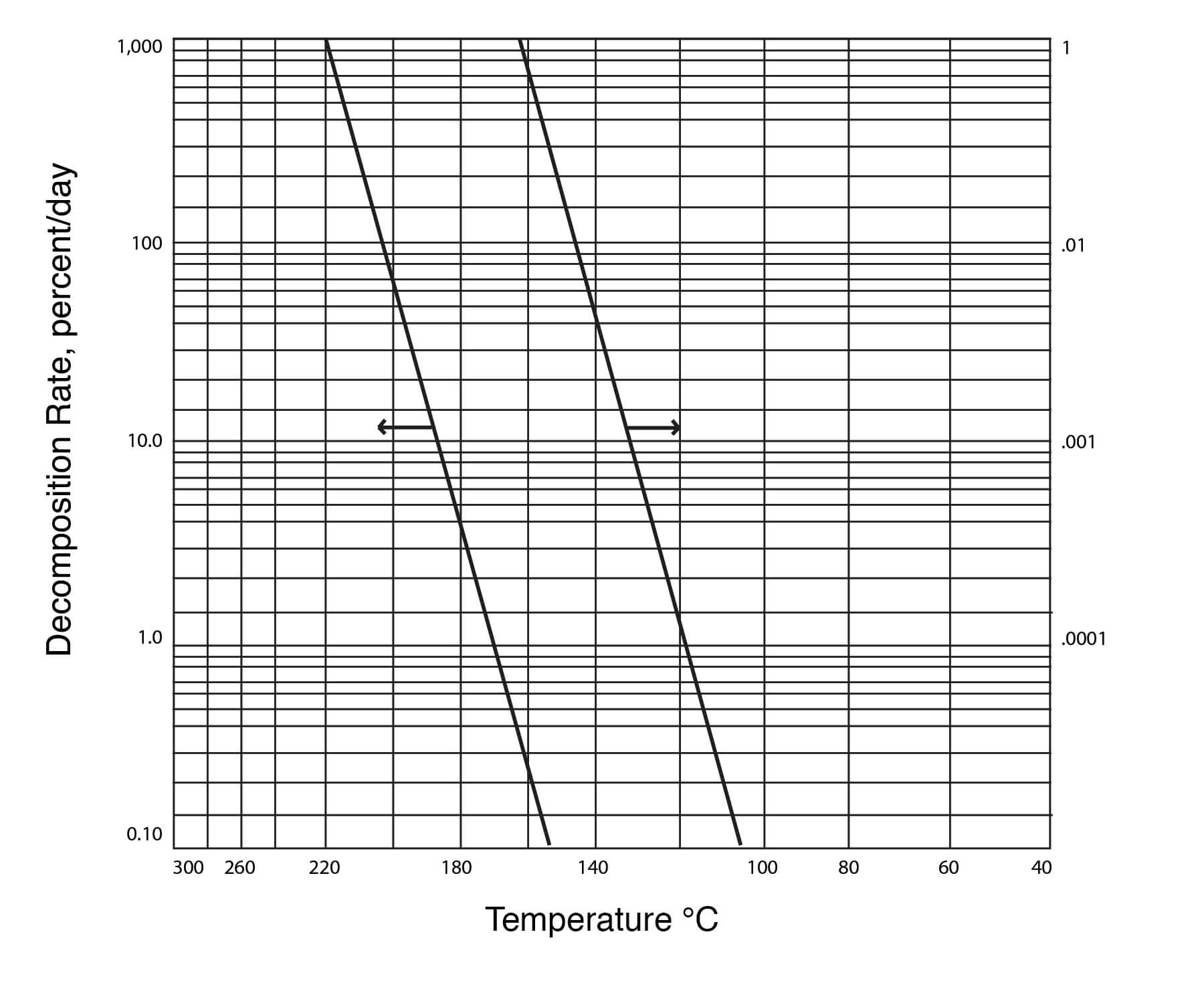
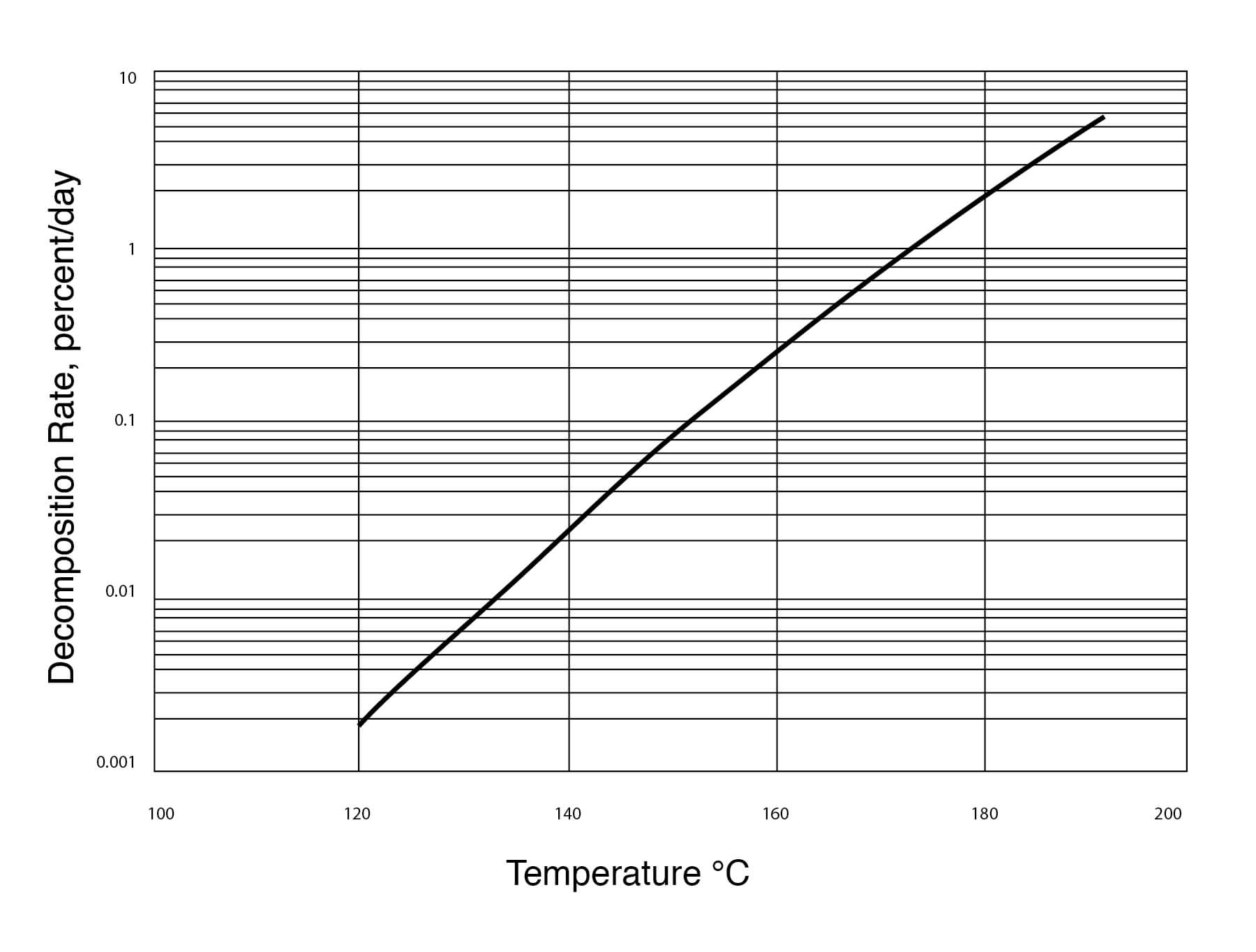
Study on Autocatalytic Decomposition of Dimethyl Sulfoxide (DMSO) | Organic Process Research & Development
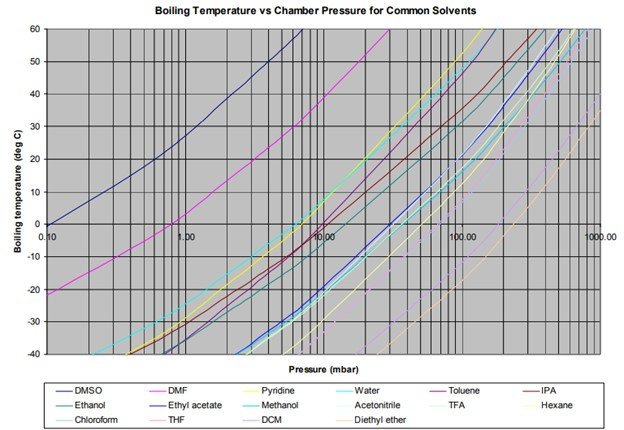
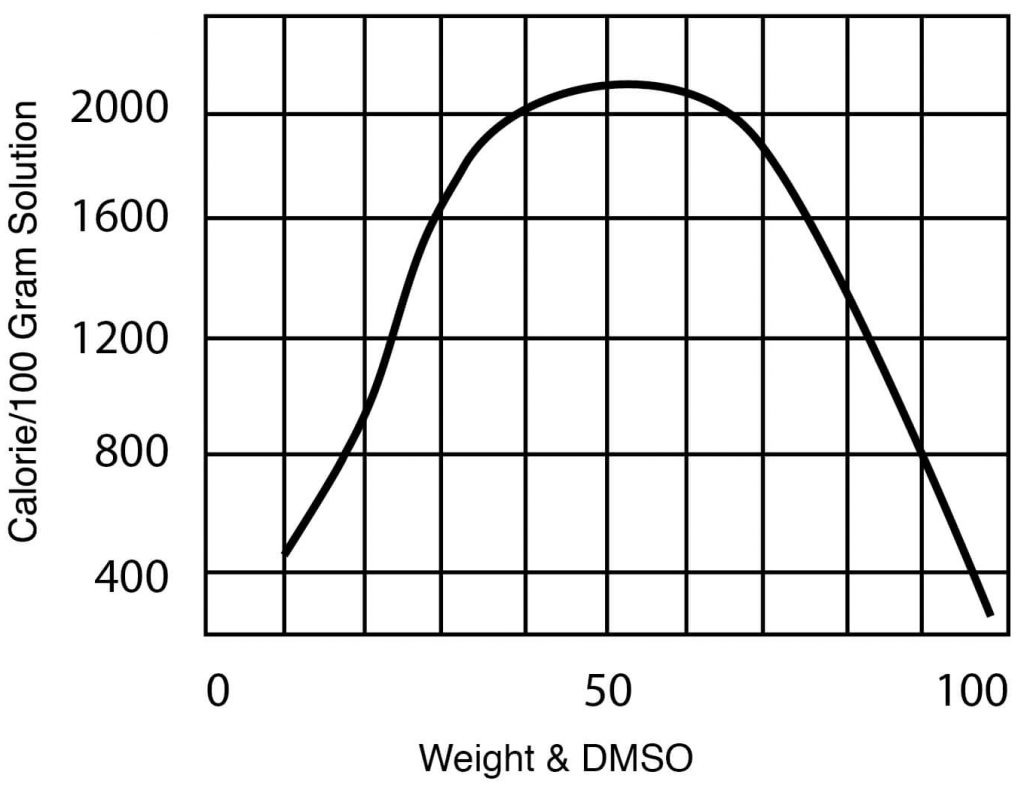
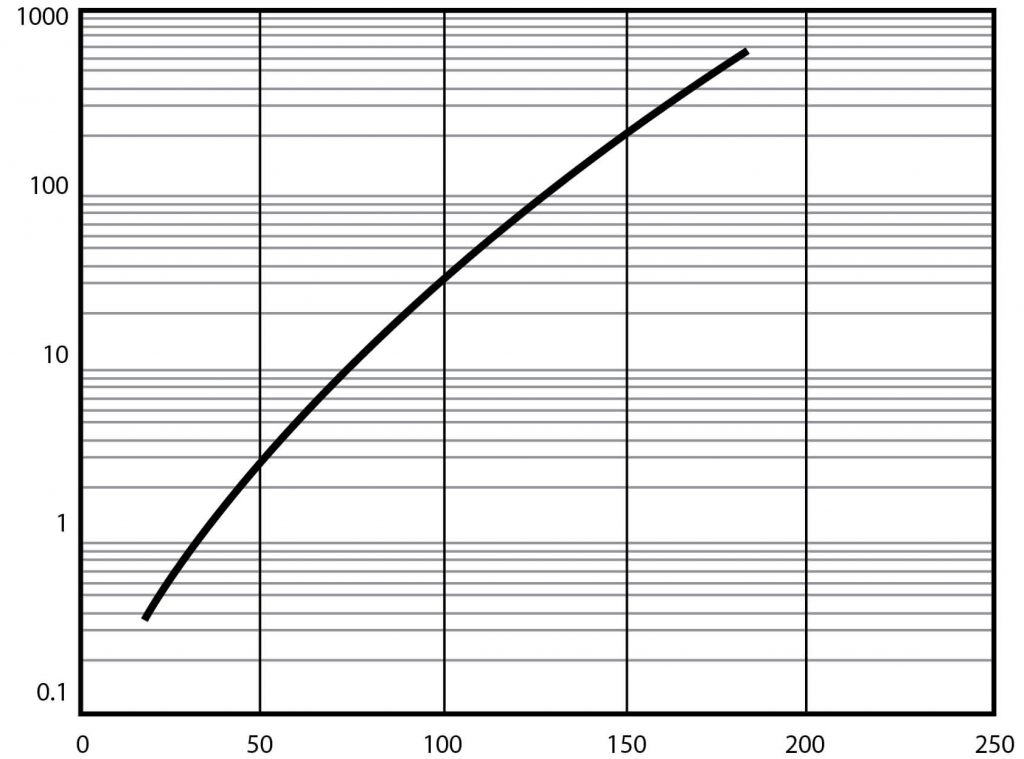
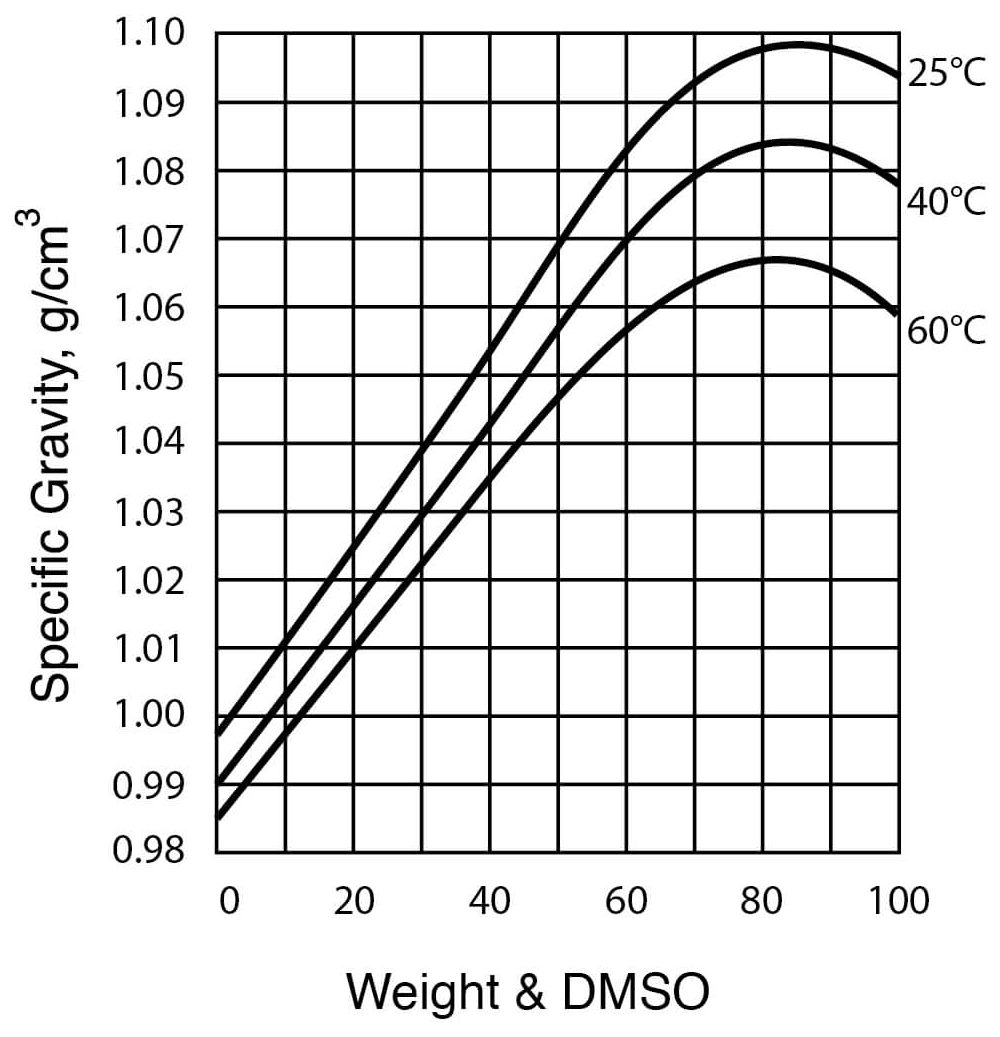
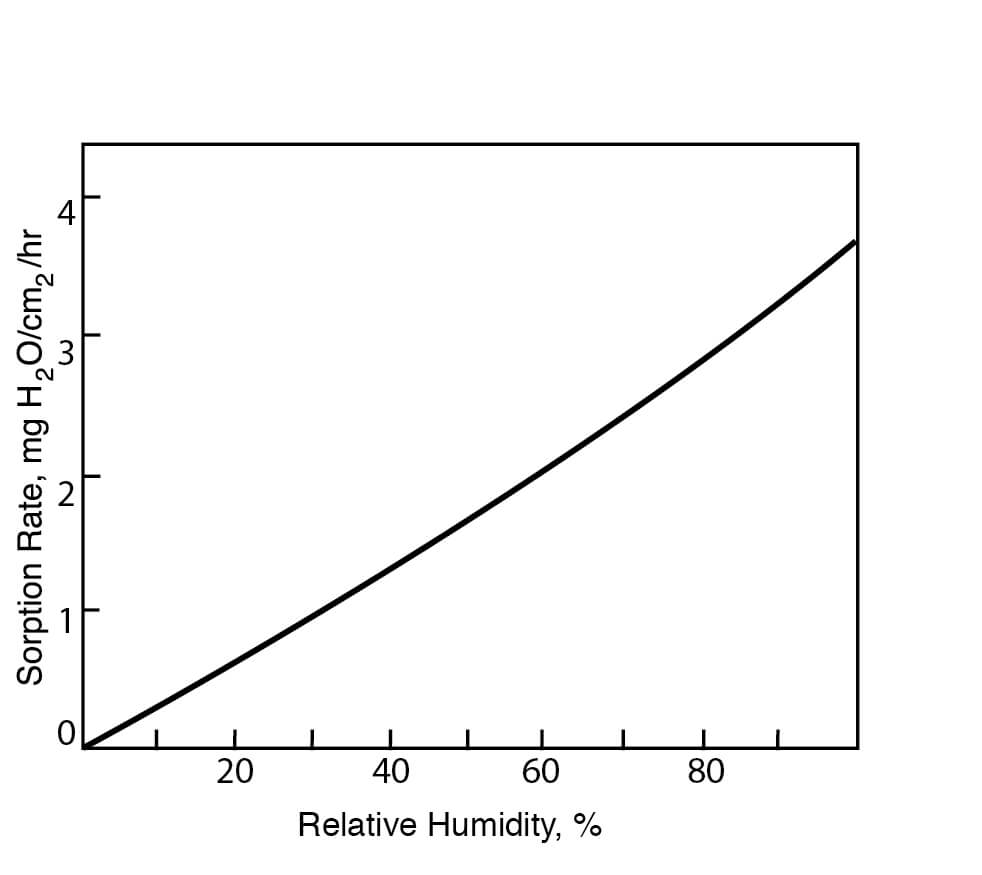
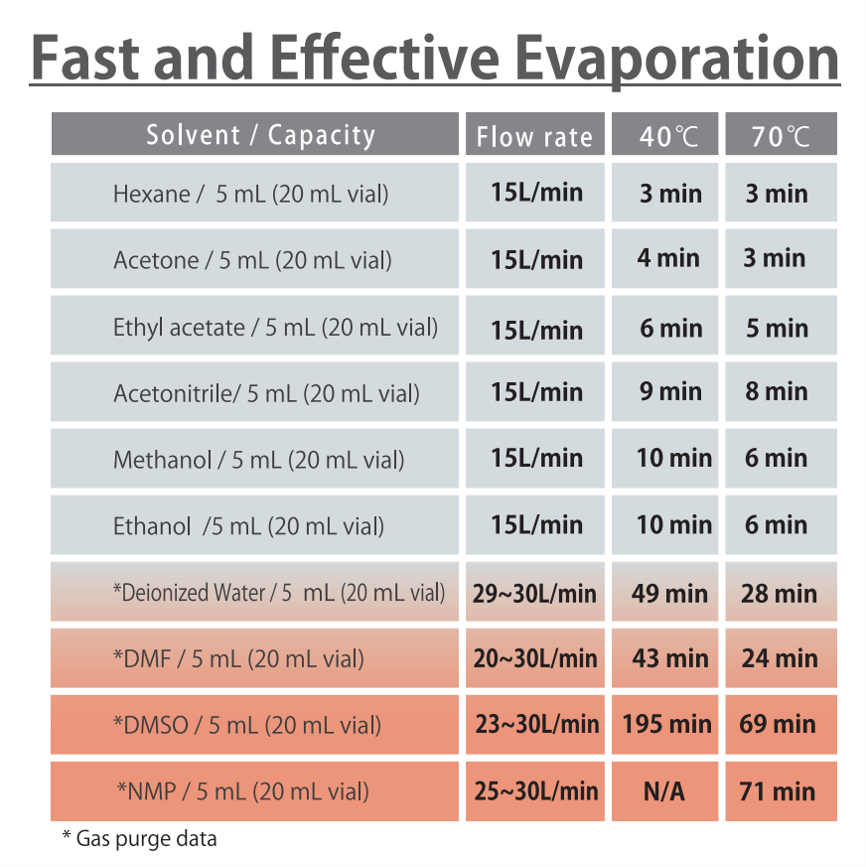
Typical DMSO Properties .................................................................... page 2 Vapor Pressure vs. Tempera
![PDF] Vapor pressure, density, viscosity and refractive index of dimethyl sulfoxide + 1,4-dimethylbenzene system | Semantic Scholar PDF] Vapor pressure, density, viscosity and refractive index of dimethyl sulfoxide + 1,4-dimethylbenzene system | Semantic Scholar](https://d3i71xaburhd42.cloudfront.net/c29c81c88658c9e680cd02ffa38c696a058a87b1/2-Table2-1.png)
PDF] Vapor pressure, density, viscosity and refractive index of dimethyl sulfoxide + 1,4-dimethylbenzene system | Semantic Scholar