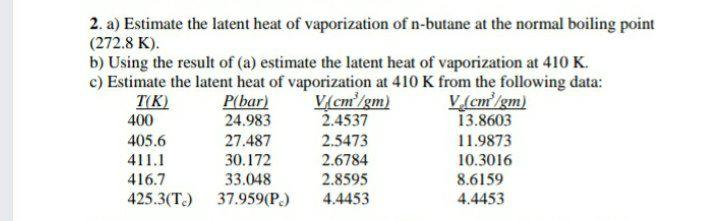
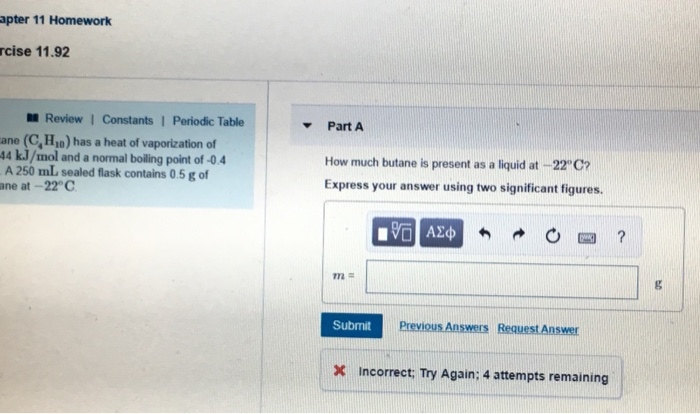
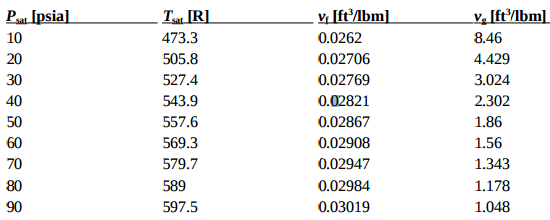
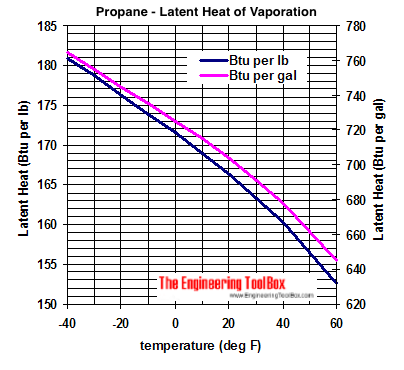
Melting Point, Boiling Point, and Heat of Vaporization of Some Common... | Download Scientific Diagram

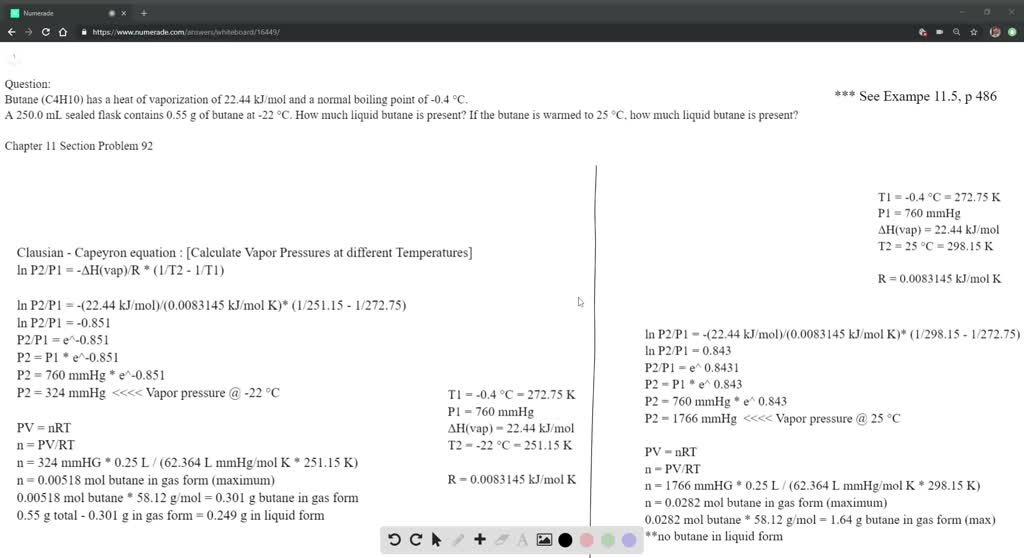
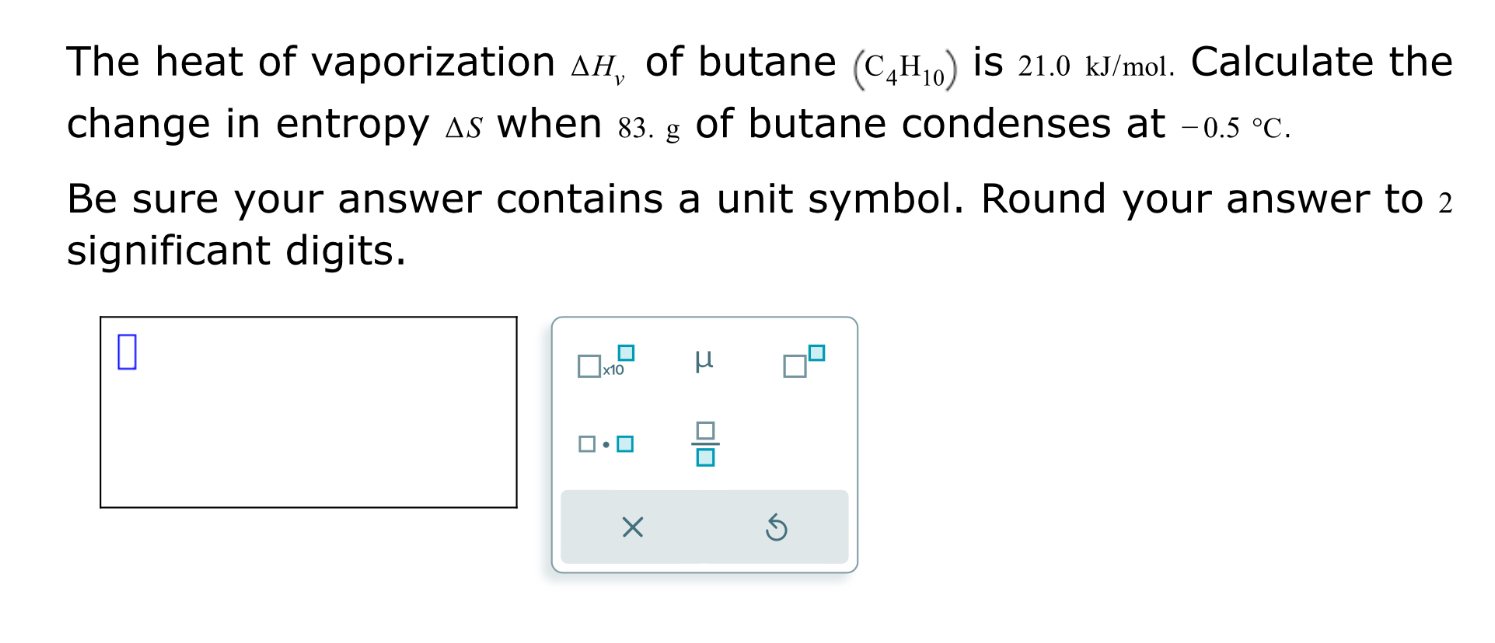
SOLVED: The heat of vaporization ΔHv of butane (C4H10) is 21.0 kJ/mol. Calculate the change in entropy ΔS when 60 g of butane condenses at -0.5°C. Be sure your answer contains a

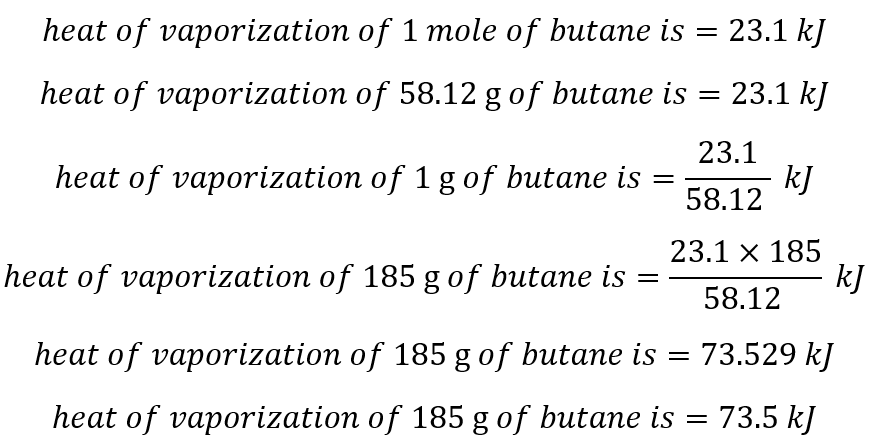
SOLVED: How much energy is required to vaporize 185 g of butane at its boiling point? The heat of vaporization for butane is 23.1 kJ/mol.